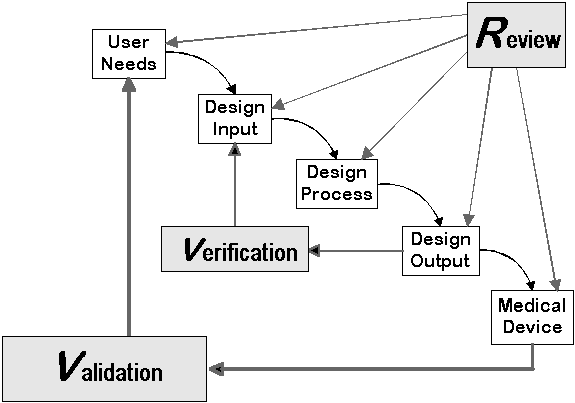
Test Method Development
Industry Drivers
Due to changing Market pressures in the Life Sciences arena, there is an increased focus in the use of Advanced Technologies to shorten the Product Development Lifecycle and deliver improved clinical outcomes to patients.
One of the key requirements that run in parallel with these developments is the ability to develop the necessary Test Methods and Analysis Procedures to demonstrate that the technologies are effectively controlled.
Glantreo Capabilities
Glantreo has experience in developing such Test Methods and Analysis Procedures in the space of Materials related technologies. The Scientific Team within Glantreo have the ability to:
- Collaborate with companies to support Test Method Strategies of Advanced Technologies
- Develop test methods for technologies that do not have already defined test methods. This service includes the identification, proof of concept and implementation of such testing regimes
- Work with the Process Development functions in Organisations to understand their Processes to develop the necessary Test Methods to support Regulatory Submissions
- Carry out the necessary testing procedures as part of the on-going control of the developed processes as well as on-going testing regimes for new technologies to support new product introduction
- Use the experience gained in working with many ISO and ASTM Standards with particular emphasis on ASTM F67, ASTM E354, ASTM 1926, ASTM 1147, and ASTM 1185