Applications and Uses of Functionalised Magnetic Silica Nanoparticles
Introduction
Glantreo’s magnetic particles are the product of many years working with silica particles and chemistries as well as the desire to deliver the superior quality products for bio-analysis and separations. These particles available in 2 different particle sizes (500nm and 1000nm) can be supplied as unfunctionalized, Amine functionalised, carboxyl functionalised and protein A functionalised. The particles are supplied in aqueous format.
The particles themselves are monodispersed magnetite particles coated with a thin layer of silica that allows functionalisation with amine and carboxyl groups as well as functionalisation with protein A. The particle size of 0.5-1um produced the optimal size for attaching bio molecules for research, purification, and samples preparation studies. Glantreos magnetic beaded are completely spherical unlike other magnetic beads and the smooth surface eliminate the carryover carryover of impurities common to rough-surfaced beads.
Because the silica magnetic beads are spherical and uniform, DNA/RNA or protein purification can be performed with outstanding efficiency and reproducibility. High magnetization (60 emu/g) and strong binding capacity giving fast magnetic response (~5 secs) and shorten time required for magnetic steps during isolation. The magnetic property of the bead allows an external magnetic field to be applied for quick separation. Our magnetic beads remain well suspended and dispersed for over 60 mins providing good handling and suitability for automation.
Magnetic beads can be used in purification of biosimilar and antibody drugs. Magnetic beads have been developed as an alternative to polymeric resins. With the correct functionality applied to the magnetic particle magnetic beads can bind to a host of biomolecules which can then be easily separated and isolated.
Features and benefits of Glantreo magnetic silica platform;
+ Range of Functionalities available (Raw, NH2, COOH & Protein A)
+ Spherical and highly magnetic (60emu)
+ High surface to volume ratio for rapid and effective purification
+ Higher surface area that competitors allowing higher binding capacities
+ Fast binding
+ Reduced Non specific binding due to optimised surface chemistry
+ Easily application to automated systems
Application Area 1: Magnetic Antibody Separation (IgG2)
Antibodies are commonly purified from serum, ascites fluid and cell culture medium using Protein A based beads in a column chromatography format. Although well established, column chromatography has several limitations, including a need for column packing and expensive instrumentation, the inability to handle multiple samples in parallel, the inability to handle small sample volumes and the elution of a relatively dilute antibody.
Magnetic bead‐based affinity purification eliminates or reduces many of these challenges and offers several advantages over conventional column‐based purification methods. Magnetic beads are easy-to-use, can process biological samples containing particulate material and do not require expensive chromatography equipment.
In addition, large numbers of samples can be handled in parallel, and the purification process can be automated. Finally, it is easy to handle small amounts of beads, making it straight forward to process small sample volumes and concentrate purified antibody. Shown in the figure below is data for the isolation of IGG2 using Glantreo Protein A coated magnetic bead.
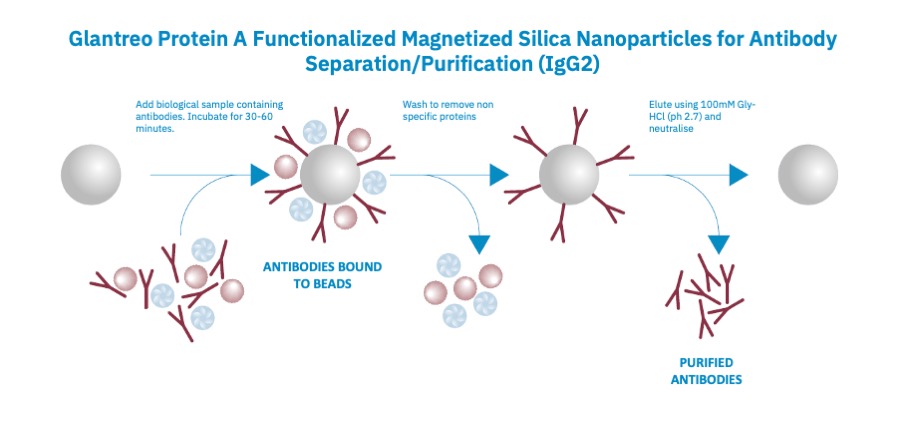
Figure 1: Schematic of usage of Glantreo protein A functionalised magnetised silica nanoparticles
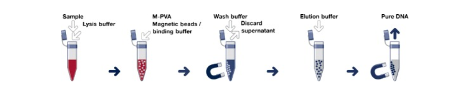
Application Area 2: Magnetic DNA/RNA Purification
Un-Functionalized (or naked) magnetic beads can be utilised for the integrated DNA and RNA (nucleic acid) extraction. Nucleic acid separation is an increasingly important tool for molecular biology. Before modern technologies could be used, nucleic acid separation had been a time- and work-consuming process based on several extraction and centrifugation steps, often limited by small yields and low purities of the separation products, and not suited for automation and up-scaling.
During the last few years magnetic particles were developed to aid the facile extraction of nucleic acids . Together with an appropriate buffer system, they allow for the quick and efficient purification directly after their extraction from crude cell extracts. Magnetic separation of nucleic acids has several advantages compared to other techniques used for the same purpose. Nucleic acids can be isolated directly from crude sample materials such as blood, tissue homogenates, cultivation media, water, etc.
The particles are used in batch processes where there are hardly any restrictions with respect to the sample volumes. Magnetic Silica particles serve as a basis of various automated low- to high-throughput procedures that allow to save time and money. Centrifugation steps can be avoided and the risk of cross-contamination when using traditional methods is no longer encountered.
Application Area 3: Magnetic Protein Separation
Isolation, separation and purification of various types of proteins and peptides, as well as of other specific molecules, is used in almost all branches of biosciences and biotechnologies. Separation science and technology is thus very important area necessary for further developments in bio-oriented research and technology. New separation techniques, capable of treating dilute solutions or solutions containing only minute amounts of target molecules in the presence of vast amounts of accompanying compounds in both small and large-scale processes, even in the presence of particulate matter, are necessary.
In the area of biosciences and biotechnology the isolation of proteins and peptides is usually performed using variety of chromatography, electrophoretic, ultrafiltration, precipitation and other procedures, affinity chromatography being one of the most important techniques. Affinity ligand techniques represent currently the most powerful tool available to the downstream processing both in term of their selectivity and recovery. The strength of column affinity chromatography has been shown in thousands of successful applications, especially in the laboratory scale.
However, the disadvantage of all standard column liquid chromatography procedures is the impossibility of the standard column systems to cope with the samples containing particulate material so they are not suitable for work in early stages of the isolation/purification process where suspended solid and fouling components are present in the sample. In this case magnetic affinity, ion-exchange, hydrophobic or adsorption batch separation processes, applications of magnetically stabilized fluidized beds or magnetically modified two-phase systems have shown their usefulness.
Magnetic carriers bearing an immobilized affinity or hydrophobic ligand or ion-exchange groups, or magnetic biopolymer particles having affinity to the isolated structure, are mixed with a sample containing target compound(s). Samples may be crude cell lysates, whole blood, plasma, ascites fluid, milk, whey, urine, cultivation media, wastes from food and fermentation industry and many others.
Following an incubation period when the target compound(s) bind to the magnetic particles the whole magnetic complex is easily and rapidly removed from the sample using an appropriate magnetic separator. After washing out the contaminants, the isolated target compound(s) can be eluted and used for further work. The magnetic separation techniques are also the basis of various automated procedures, especially magnetic-particle based immunoassay systems for the determination of a variety of analytes, among them proteins and peptides. Several automated systems for the separation of proteins or nucleic acids have become available recently.
Magnetic separation is usually very gentle to the target proteins or peptides. Even large protein complexes that tend to be broken up by traditional column chromatography techniques may remain intact when using the very gentle magnetic separation procedure [2]. Both the reduced shearing forces and the higher protein concentration throughout the isolation process positively influence the separation process.
Separation of target proteins using standard chromatography techniques often leads to the large volume of diluted protein solution. In this case appropriate magnetic particles can be used for their concentration instead of ultrafiltration, precipitation etc.
Application 4: Magnetic Cell Isolation
The separation and sorting of biological cells is critical to a variety of biomedical applications including diagnostics, therapeutics, and fundamental cell biology. As samples of interest are often heterogeneous populations of cells that are in culture or that comprise a tissue, techniques to isolate specific cells are essential for understanding how cells function and respond to various stimuli.
Blood, for example, is an extremely information-rich and easily accessible tissue that is a complex blend of cells; accurate analysis of blood character and condition requires isolation of a few desired cells. Effective cell sorting to support numerous biomedical pursuits relies upon optimal matching between the target cell attributes, desired outcomes, and the parameters of the sorting technique. Numerous cell isolation and sorting techniques have been developed for benchtop and clinical settings that are based on either physical properties of the cell, such as density or size, or on cell affinity that describes electric, magnetic or adhesive properties specific to each cell type.
Standard techniques for the separation of cells include processing steps of filtration, centrifugation and sedimentation, which are carried out either in a batch or in a continuous manner and can be easily translated to large-scale operation. However, in situations where cell size or density differences are not significant, effective cell separation is impeded in these techniques and other methods must be employed, including fluorescence activated cell sorting (FACS) and magnetic activated cell sorting (MACS).
In this context, magnetic particles —nanoparticles (mean diameter 10 – 100 nm), sub-micron particles (0.1 – 1 microns), and microparticles (mean diameter 1 – 50 microns) — have been an important component of cell separation techniques in both biomedical research and in clinical medicine.
