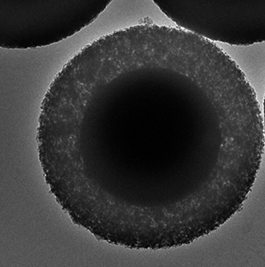
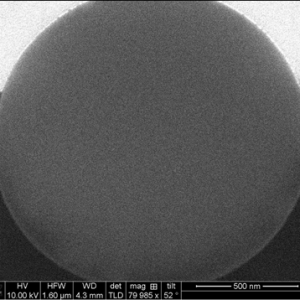
SOLAS™ is a proprietary process for the world’s first monodense, monodisperse fully porous silica particle for applications in chromatography, drug delivery, diagnostics, energy etc. These particles represent the cutting edge in silica technology and are unique in that they combine particle monodispersivity, and monodensity along with and a wide range of pore sizes through Glantreo’s Controlled & Stable Porosity (CSP) process. These innovative particles offer seamless bonding and functionalisation capabilities. Additionally, the SOLAS™ particles represent a media that is easier to pack with a reduced failure rate in packing.
❖Supports for metal and enzyme catalysts
❖Drug Delivery and controlled drug release
❖Water and air filtration
❖Sensing Devices due to high customisability
❖Insulation Materials due to low thermal conductivity

| Particle Size | 150nm, 200nm, 350nm, 500nm, 750nm, 1.0um, 1.5um, 1.7um, 1.9um, 3.0um, 5.0um, 10um, 25um. |
| Pore Size | 20A, 40A, 100A, 500A, 1000A, others upon request. |
| Functionalisation | Raw Silica, c18, c8, c4, Carboxyl, Streptavidin, Amine, Thiol. You can see the price for the Raw Chemistry versions in the shopping cart. Please Ask and Expert for pricing for other functional groups. |
| Pack Size | 5g |
| d90/d10 | < 1.3 |
If you have published and cited Glantreo’s materials then click here to let us know.
Clifford A. Barnes, Andreas Elsaesser, Joanna Arkusz, Anna Smok, Jadwiga Palus, Anna Lesniak, Anna Salvati, John P. Hanrahan, Wim H. de Jong, Elzbieta Dziubaltowska, Maciej St?pnik, Konrad Rydzynski, George McKerr, Iseult Lynch, Kenneth A. Dawson and C. Vyvyan Howard, Reproducible Comet Assay of Amorphous Silica Nanoparticles Detects No Genotoxicity. Nano Letters, 2008, 8 (9), pp 3069–3074. doi: 10.1021/nl801661w http://pubs.acs.org/doi/abs/10.1021/nl801661w#citing
Makoto Ema∗, Norihiro Kobayashi, Masato Naya, Sosuke Hanai, Junko Nakanishi, Reproductive and developmental toxicity studies of manufactured nanomaterials, Reproductive Toxicology 30 (2010) 343–352
Iseult Lynch, Sara Linse, C Vyvyan Howard, Maciej Stepnik, Konrad Rydzynski, John Hanrahan, Wim de Jong, Dominique Langevin, Joachim Rädler, Wolfgang Parak, Yuri Volkov, Marek Radomski, Robert Thomas, Jacob Klein, Andrew A Barron, Colin Janssen, Fiona M Lyons, Francis Quinn, Bert Swennen, Peter Cuypers, Angela Duffy and Kenneth A Dawson, NANOINTERACT: A rational approach to the interaction between nanoscale materials and living matter, Journal of Physics: Conference Series Volume 170 Number 1.doi:10.1088/1742-6596/170/1/012040 http://iopscience.iop.org/1742-6596/170/1/012040/cites
Bashir Mustafa Mohamed, Navin Kumar Verma, Adriele Prina-Mello, Yvonne Williams, Anthony M Davies, Gabor Bakos, Laragh Tormey, Connla Edwards, John Hanrahan, Anna Salvati, Iseult Lynch, Kenneth Dawson, Dermot Kelleher,and Yuri Volkov, Activation of stress-related signalling pathway in human cells upon SiO2 nanoparticles exposure as an early indicator of cytotoxicity. Journal of Nanobiotechnology. 2011;9:29. doi:10.1186/1477-3155-9-29.http://www.jnanobiotechnology.com/content/9/1/29
Margriet V.D.Z. Park a,b,⁎, Wijtske Annema a, Anna Salvati c, Anna Lesniak c, Andreas Elsaesser d, Clifford Barnes d, George McKerr d, C. Vyvyan Howard d, Iseult Lynch c, Kenneth A. Dawson c, Aldert H. Piersma a,e, Wim H. de Jong a, In vitro developmental toxicity test detects inhibition of stem cell differentiation by silica nanoparticles, Toxicology and Applied Pharmacology 240 (2009) 108–116
Markus Roller, In vitro genotoxicity data of nanomaterials compared to carcinogenic potency of inorganic substances after inhalational exposure, Mutation Research 727 (2011) 72–85
Mostafa Yazdimamaghani, PhDa,b, Philip J. Moos, PhDb,c, Marina A. Dobrovolskaia, PhDd, Hamidreza Ghandehari, PhD, Genotoxicity of amorphous silica nanoparticles: Status and prospects, Nanomedicine: Nanotechnology, Biology, and Medicine 16 (2019) 106–125