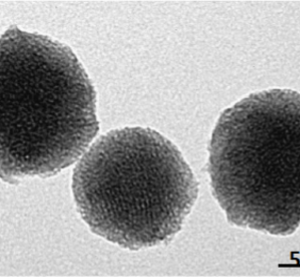
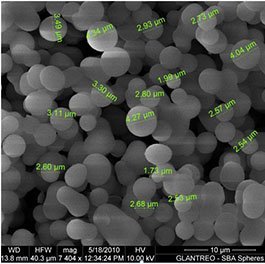
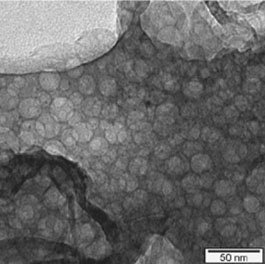
MCM are customisable mesoporous silica, with uniform cylindrical pores in either a hexagonal arrangement (MCM-41) or cubic arrangement (MCM-48). Pore sizes can be adjusted to be from 20A to 500A lending these materials has a high surface area and large pore volume, perfect for loading large molecules such as proteins, or metals for catalysis, which may not be able to enter smaller pores of zeolites or other mesoporous silica. Additionally, MCM can be functionalised with amine groups, thiols, doped with aluminium and their hydrophobicity/hydrophilicity can be tuned by addition of silanol groups.
Glantreo has vast knowledge in the manufacture of Mesoporous Silica (MS). For almost 5 years we have supplied large quantities (up to kg quantites) of Mesoporous Silica to our Researcher and Industry partners.
In addition to the raw and functionalised materials we also offer all variations in pelletised formats. The pellets are available in 2mm x 5mm. Please enquire to discuss your requirements in more detail.

❖ Chromatography
❖ Drug Delivery
❖ Hard Templating
❖ Immobilization of Bioactive Molecules
❖ Encapsulation
❖ Polymer Reinforcement
❖ Catalysis



| Particle Type | MCM-41 | MCM-48 |
|---|---|---|
| Purity | 99.99% | 99.99% |
| Particle Sizes | 100um* | 10um & 150um* (MCM-48 Aluminosilicate) |
| Particle Morphology | Hexagonal | Cubic |
| Pore Sizes | 25A & 30A* (MCM41-Aluminosilicate) | 25A & 30A* (MCM41-Aluminosilicate) |
| Formats | Powders & Pellets | Powders & Pellets |
| Chemistries | Raw, Amine, Thiol | Raw, Amine, Thiol |
| * Indicative values. The materials specification is a range in value. | ||
| Product Code | Type | Pore Morphology | Format | Particle Size | Pore Size | Pack Size | Chemistry |
|---|---|---|---|---|---|---|---|
| PMCM4110025R-5 | MCM-41 | Hexaganol | Powder | 100um | 25A | 5g | Silanol/Raw |
| PMCM4110025NH1-5 | MCM-41 | Hexaganol | Powder | 100um | 25A | 5g | Amine |
| PMCM4110025SH1-5 | MCM-41 | Hexaganol | Powder | 100um | 25A | 5g | Thiol |
| PMCM4110025R-50 | MCM-41 | Hexaganol | Powder | 100um | 25A | 50g | Silanol/Raw |
| PMCM4110025NH1-50 | MCM-41 | Hexaganol | Powder | 100um | 25A | 50g | Amine |
| PMCM4110025SH1-50 | MCM-41 | Hexaganol | Powder | 100um | 25A | 50g | Thiol |
| PMCM41AL10030R-50 | MCM-41 Aluminosilicate | Hexaganol | Powder | 100um | 30A | 50g | Silanol/Raw |
| PMCM41AL10030R-5 | MCM-41 Aluminosilicate | Hexaganol | Powder | 100um | 30A | 5g | Silanol/Raw |
| PMCM41AL10030NH1-5 | MCM-41 Aluminosilicate | Hexaganol | Powder | 100um | 30A | 5g | Amine |
| PMCM41AL10030SH1-5 | MCM-41 Aluminosilicate | Hexaganol | Powder | 100um | 30A | 5g | Thiol |
| PMCM41AL10030NH1-50 | MCM-41 Aluminosilicate | Hexaganol | Powder | 100um | 30A | 50g | Amine |
| PMCM41AL10030SH1-50 | MCM-41 Aluminosilicate | Hexaganol | Powder | 100um | 30A | 50g | Thiol |
| PELMCM4110025R-5 | MCM-41 Pellets | Hexaganol | Pellets | 100um | 25A | 5g | Silanol/Raw |
| PMCM481025R-5 | MCM-48 | Cubic | Powder | 10um | 25A | 5g | Silanol/Raw |
| PMCM481025NH1-5 | MCM-48 | Cubic | Powder | 10um | 25A | 5g | Amine |
| PMCM481025SH1-5 | MCM-48 | Cubic | Powder | 10um | 25A | 5g | Thiol |
| PMCM481025R-50 | MCM-48 | Cubic | Powder | 10um | 25A | 50g | Silanol/Raw |
| PMCM481025NH1-50 | MCM-48 | Cubic | Powder | 10um | 25A | 50g | Amine |
| PMCM481025SH1-50 | MCM-48 | Cubic | Powder | 10um | 25A | 50g | Thiol |
| PMCM48AL15030NH1-5 | MCM-48 Aluminosilicate | Cubic | Powder | 150um | 30A | 5g | Amine |
| PMCM48AL15030R-5 | MCM-48 Aluminosilicate | Cubic | Powder | 150um | 30A | 5g | Silanol/Raw |
| PMCM48AL15030SH1-5 | MCM-48 Aluminosilicate | Cubic | Powder | 150um | 30A | 5g | Thiol |
| PMCM48AL15030R-50 | MCM-48 Aluminosilicate | Cubic | Powder | 150um | 30A | 50g | Silanol/Raw |
| PMCM48AL15030NH1-50 | MCM-48 Aluminosilicate | Cubic | Powder | 150um | 30A | 50g | Amine |
| PMCM48AL15030SH1-50 | MCM-48 Aluminosilicate | Cubic | Powder | 150um | 30A | 50g | Thiol |
| PELMCM481025R-5 | MCM-48 Pellets | Cubic | Pellets | 10um | 25A | 5g | Silanol/Raw |
If you have published and cited Glantreo’s materials then click here to let us know.
Noreldeen H. Abdallah, Miriam Schlumpberger, Darragh A. Gaffney, John P. Hanrahan, Joseph M. Tobin, Edmond Magner, Comparison of mesoporous silicate supports for the immobilisation and activity of cytochrome c and lipase, Journal of Molecular Catalysis B: Enzymatic, Volume 108, October 2014, Pages 82-88, ISSN 1381-1177http://www.sciencedirect.com/science/article/pii/S1381117714001805
Robert J. Ahern, John P. Hanrahan, Joseph M. Tobin, Katie B. Ryan, Abina M. Crean, Comparison of fenofibrate–mesoporous silica drug-loading processes for enhanced drug delivery, European Journal of Pharmaceutical Sciences 50 (2013) 400–409
Robert J. Ahern, John P. Hanrahan, Joseph M. Tobin, Katie B. Ryan, Abina M. Crean, Comparison of fenofibrate–mesoporous silica drug-loading processes for enhanced drug delivery, European Journal of Pharmaceutical Sciences, Volume 50, Issues 3–4, 20 November 2013, Pages 400-409, ISSN 0928-0987 http://www.sciencedirect.com/science/article/pii/S0928098713003400
Davide Barreca, Mark P. Copley, Andrew E. Graham, Justin D. Holmes, Michael A. Morris, Roberta Seraglia, Trevor R. Spalding, Eugenio Tondello, Methanolysis of styrene oxide catalysed by a highly efficient zirconium-doped mesoporous silica, Applied Catalysis A: General, Volume 304, 10 May 2006, Pages 14-20, ISSN 0926-860Xhttp://www.sciencedirect.com/science/article/pii/S0926860X06001013
Paul Delaney, Healy RM, Hanrahan JP, Gibson LT, Wenger JC, Morris MA, Holmes JD. Porous silica spheres as indoor air pollutant scavengers. Journal of Environmental Monitoring. 2010 Dec;12(12):2244-51. doi: 10.1039/c0em00226g.http://www.ncbi.nlm.nih.gov/pubmed/20941430
Tahnee J. Deninga,1, Dmitry Zemlyanovb, Lynne S. Taylora, Application of an adsorption isotherm to explain incomplete drug release from ordered mesoporous silica materials under supersaturating conditions, Journal of Controlled Release 307 (2019) 186–199
Jessica Fordea, Alex Vakurovb, Tim D. Gibsonb, Paul Millnerb, Mícheál Whelehana, Ian W. Marisona, Ciarán Ó’Fágáina, Chemical modification and immobilisation of lipase B from Candida antarctica onto mesoporous silicates, Journal of Molecular Catalysis B: Enzymatic 66 (2010) 203–209
Tomer Lapidot, Omar K. Matar, Jerry Y.Y. Heng, Calcium sulphate crystallisation in the presence of mesoporous silica particles: Experiments and population balance modelling, Chemical Engineering Science 202 (2019) 238–249
Carol A. McCarthy, Waleed Faisal, Joseph P. O’Shea, Colm Murphy, Robert J. Aherne, Katie B. Ryan, Brendan T. Griffin, Abina M. Crean , In vitro dissolution models for the prediction of in vivo performance of an oral mesoporous silica formulation, Journal of Controlled Release, Volume 250, 28 March 2017, Pages 86-95
K. Lamb, R.A. Mole, D. Yu, R. de Marco, J.R. Bartlett, S. Windsor, S.P. Jiang, J. Zhang, V.K. Peterson, Proton dynamics in phosphotungstic acid impregnated mesoporous silica proton exchange membrane materials, Green Energy & Environment (2017), doi: 10.1016/ j.gee.2017.06.007.
Mareike Siebert∗, Thorben Detering, Ralf G. Berger, An immobilized fungal chlorogenase rapidly degrades chlorogenic acid in a T coffee beverage without altering its sensory properties, LWT – Food Science and Technology 115 (2019) 108426
Sugata P. Tan*, Elizabeth Barsotti, Mohammad Piri, Application of material balance for the phase transition of fluid mixtures confined in nanopores, Fluid Phase Equilibria 496 (2019) 31e41
Laura J. Waters a,⇑, Talib Hussain a, Gareth Parkes a, John P. Hanrahan b, Joseph M. Tobin, Inclusion of fenofibrate in a series of mesoporous silicas using microwave irradiation, European Journal of Pharmaceutics and Biopharmaceutics 85 (2013) 936–941
Laura J. Waters, Talib Hussain, Gareth Parkes, John P. Hanrahan, Joseph M. Tobin, Inclusion of fenofibrate in a series of mesoporous silicas using microwave irradiation, European Journal of Pharmaceutics and Biopharmaceutics, Volume 85, Issue 3, Part B, November 2013, Pages 936-941, ISSN 0939-6411http://www.sciencedirect.com/science/article/pii/S0939641113002816